Page 6 - An ultrasound-driven immune-boosting molecular machine for systemic tumor suppression
P. 6
SCIENCE ADVANCES | RESEARCH ARTICLE
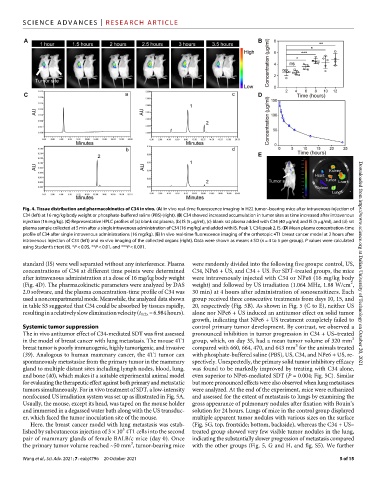
Fig. 4. Tissue distribution and pharmacokinetics of C34 in vivo. (A) In vivo real-time fluorescence imaging in H22 tumor–bearing mice after intravenous injection of
C34 (left) at 16 mg/kg body weight or phosphate-buffered saline (PBS) (right). (B) C34 showed increased accumulation in tumor sites as time increased after intravenous
injection (16 mg/kg). (C) Representative HPLC profiles of (a) blank rat plasma, (b) IS (5 g/ml), (c) blank rat plasma added with C34 (40 g/ml) and IS (5 g/ml), and (d) rat
plasma sample collected at 5 min after a single intravenous administration of C34 (16 mg/kg) and added with IS. Peak 1, C34; peak 2, IS. (D) Mean plasma concentration-time
profile of C34 after single intravenous administrations (16 mg/kg). (E) In vivo real-time fluorescence imaging of the orthotopic 4T1 breast cancer model at 2 hours after
intravenous injection of C34 (left) and ex vivo imaging of the collected organs (right). Data were shown as means ± SD (n = 4 to 5 per group). P values were calculated
using Student’s t test (B). *P < 0.05, **P < 0.01, and ***P < 0.001.
standard (IS) were well separated without any interference. Plasma were randomly divided into the following five groups: control, US, Downloaded from https://www.science.org at Dalian University of Technology on October 20, 2021
concentrations of C34 at different time points were determined C34, NPe6 + US, and C34 + US. For SDT-treated groups, the mice
after intravenous administration at a dose of 16 mg/kg body weight were intravenously injected with C34 or NPe6 (16 mg/kg body
2
(Fig. 4D). The pharmacokinetic parameters were analyzed by DAS weight) and followed by US irradiation (1.064 MHz, 1.88 W/cm ,
2.0 software, and the plasma concentration-time profile of C34 was 30 min) at 4 hours after administration of sonosensitizers. Each
used a noncompartmental mode. Meanwhile, the analyzed data shown group received three consecutive treatments from days 10, 15, and
in table S3 suggested that C34 could be absorbed by tissues rapidly, 20, respectively (Fig. 5B). As shown in Fig. 5 (C to E), neither US
resulting in a relatively slow elimination velocity (t 1/2z = 6.984 hours). alone nor NPe6 + US induced an antitumor effect on solid tumor
growth, indicating that NPe6 + US treatment completely failed to
Systemic tumor suppression control primary tumor development. By contrast, we observed a
The in vivo antitumor effect of C34-mediated SDT was first assessed pronounced inhibition in tumor progression in C34 + US–treated
3
in the model of breast cancer with lung metastasis. The mouse 4T1 group, which, on day 35, had a mean tumor volume of 320 mm
3
breast tumor is poorly immunogenic, highly tumorigenic, and invasive compared with 660, 664, 470, and 643 mm for the animals treated
(39). Analogous to human mammary cancer, the 4T1 tumor can with phosphate-buffered saline (PBS), US, C34, and NPe6 + US, re-
spontaneously metastasize from the primary tumor in the mammary spectively. Unexpectedly, the primary solid tumor inhibitory efficacy
gland to multiple distant sites including lymph nodes, blood, lung, was found to be markedly improved by treating with C34 alone,
and bone (40), which makes it a suitable experimental animal model even superior to NPe6-mediated SDT (P = 0.004; Fig. 5C). Similar
for evaluating the therapeutic effect against both primary and metastatic but more pronounced effects were also observed when lung metastases
tumors simultaneously. For in vivo treatment of SDT, a low-intensity were analyzed. At the end of the experiment, mice were euthanized
nonfocused US irradiation system was set up as illustrated in Fig. 5A. and assessed for the extent of metastasis to lungs by examining the
Usually, the mouse, except its head, was taped on the mouse holder gross appearance of pulmonary nodules after fixation with Bouin’s
and immersed in a degassed water bath along with the US transduc- solution for 24 hours. Lungs of mice in the control group displayed
er, which faced the tumor inoculation site of the mouse. multiple apparent tumor nodules with various sizes on the surface
Here, the breast cancer model with lung metastasis was estab- (Fig. 5G, top, frontside; bottom, backside), whereas the C34 + US–
5
lished by subcutaneous injection of 3 × 10 4T1 cells into the second treated group showed very few visible tumor nodules in the lung,
pair of mammary glands of female BALB/c mice (day 0). Once indicating the substantially slower progression of metastasis compared
3
the primary tumor volume reached ~50 mm , tumor-bearing mice with the other groups (Fig. 5, G and H, and fig. S5). We further
Wang et al., Sci. Adv. 2021; 7 : eabj4796 20 October 2021 5 of 15