Page 9 - An ultrasound-driven immune-boosting molecular machine for systemic tumor suppression
P. 9
SCIENCE ADVANCES | RESEARCH ARTICLE
tumors might be attributed to a successful augment of DC recruit- fluorescence imaging showed higher CD80 (green) and CD86 (red)
ment, maturation, and cross-presentation, leading to strong poten- signals in the C34-treated group (Fig. 7C).
tiation of tumor antigen–specific T cell responses. We next investigated the tumor antigen cross-priming by DCs
in vitro because it is a key process for inducing the generation of
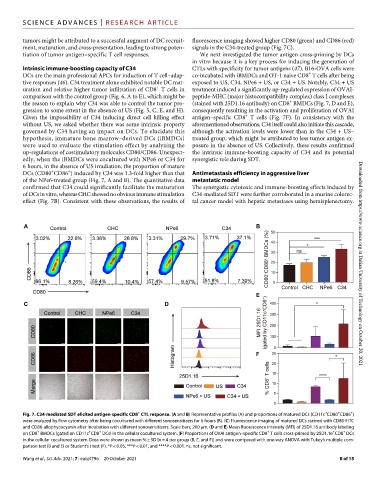
Intrinsic immune-boosting capacity of C34 CTLs with specificity for tumor antigens (47). B16-OVA cells were
+
DCs are the main professional APCs for induction of T cell–adap- co-incubated with iBMDCs and OT-1 naive CD8 T cells after being
tive responses (46). C34 treatment alone exhibited notable DC mat- exposed to US, C34, NPe6 + US, or C34 + US. Notably, C34 + US
+
uration and relative higher tumor infiltration of CD8 T cells in treatment induced a significantly up-regulated expression of OVAI-
comparison with the control group (Fig. 6, A to E), which might be peptide-MHC (major histocompatibility complex) class I complexes
+
the reason to explain why C34 was able to control the tumor pro- (stained with 25D1.16 antibody) on CD8 BMDCs (Fig. 7, D and E),
gression to some extent in the absence of US (Fig. 5, C, E, and H). consequently resulting in the activation and proliferation of OVAI
+
Given the impossibility of C34 inducing direct cell killing effect antigen–specific CD8 T cells (Fig. 7F). In consistency with the
without US, we asked whether there was some intrinsic property aforementioned observations, C34 itself could also initiate this cascade,
governed by C34 having an impact on DCs. To elucidate this although the activation levels were lower than in the C34 + US–
hypothesis, immature bone marrow–derived DCs (iBMDCs) treated group, which might be attributed to less tumor antigen ex-
were used to evaluate the stimulation effect by analyzing the posure in the absence of US. Collectively, these results confirmed
up-regulations of costimulatory molecules CD80/CD86. Unexpect- the intrinsic immune-boosting capacity of C34 and its potential
edly, when the iBMDCs were cocultured with NPe6 or C34 for synergistic role during SDT.
6 hours, in the absence of US irradiation, the proportion of mature
+
+
DCs (CD80 CD86 ) induced by C34 was 1.3-fold higher than that Antimetastasis efficiency in aggressive liver
of the NPe6-treated group (Fig. 7, A and B). The quantitative data metastatic model
confirmed that C34 could significantly facilitate the maturation The synergistic cytotoxic and immune-boosting effects induced by
of DCs in vitro, whereas CHC showed no obvious immune stimulation C34-mediated SDT were further corroborated in a murine colorec-
effect (Fig. 7B). Consistent with these observations, the results of tal cancer model with hepatic metastases using hemisplenectomy. Downloaded from https://www.science.org at Dalian University of Technology on October 20, 2021
+ + + +
Fig. 7. C34-mediated SDT elicited antigen-specific CD8 CTL response. (A and B) Representative profiles (A) and proportions of matured DCs (CD11c CD80 CD86 )
were analyzed by flow cytometry after being cocultured with different sonosensitizers for 6 hours (B). (C) Fluorescence imaging of matured DCs stained with CD80-FITC
and CD86-allophycocyanin after incubation with different sonosensitizers. Scale bars, 200 m. (D and E) Mean fluorescence intensity (MFI) of 25D1.16 antibody labeling
+
+
+
+
+
+
on CD8 BMDCs (gated on CD11c CD8 DCs) in the cellular cocultured system. (F) Proportions of OVAI antigen–specific CD8 T cells cross-primed by 25D1.16 CD8 DCs
in the cellular cocultured system. Data were shown as mean % ± SD [n = 4 per group (B, E, and F)] and were compared with one-way ANOVA with Tukey’s multiple com-
parison test (B and E) or Student’s t test (F). *P < 0.05, ***P < 0.01, and ****P < 0.001. ns, not significant.
Wang et al., Sci. Adv. 2021; 7 : eabj4796 20 October 2021 8 of 15