Page 5 - An ultrasound-driven immune-boosting molecular machine for systemic tumor suppression
P. 5
SCIENCE ADVANCES | RESEARCH ARTICLE
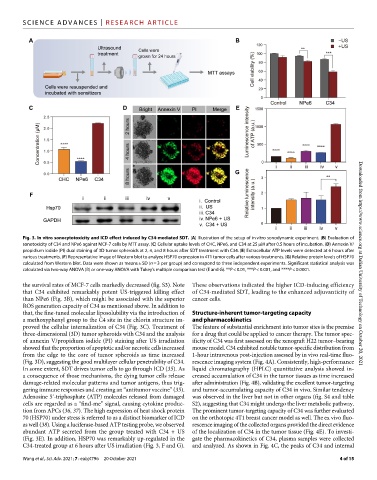
Fig. 3. In vitro sonocytotoxicity and ICD effect induced by C34-mediated SDT. (A) Illustration of the setup of in vitro sonodynamic experiment. (B) Evaluation of
sonotoxicity of C34 and NPe6 against MCF-7 cells by MTT assay. (C) Cellular uptake levels of CHC, NPe6, and C34 at 25 M after 0.5 hours of incubation. (D) Annexin V/
propidium iodide (PI) dual staining of 3D tumor spheroids at 2, 4, and 8 hours after SDT treatment with C34. (E) Extracellular ATP levels were detected at 6 hours after
various treatments. (F) Representative image of Western blot to analyze HSP70 expression in 4T1 tumor cells after various treatments. (G) Relative protein levels of HSP70
calculated from Western blot. Data were shown as means ± SD (n = 3 per group) and correspond to three independent experiments. Significant statistical analysis was Downloaded from https://www.science.org at Dalian University of Technology on October 20, 2021
calculated via two-way ANOVA (B) or one-way ANOVA with Tukey’s multiple comparison test (E and G). **P < 0.01, ***P < 0.001, and ****P < 0.0001.
the survival rates of MCF-7 cells markedly decreased (fig. S3). Note These observations indicated the higher ICD-inducing efficiency
that C34 exhibited remarkably potent US-triggered killing effect of C34-mediated SDT, leading to the enhanced adjuvanticity of
than NPe6 (Fig. 3B), which might be associated with the superior cancer cells.
ROS generation capacity of C34 as mentioned above. In addition to
that, the fine-tuned molecular liposolubility via the introduction of Structure-inherent tumor-targeting capacity
a methoxyphenyl group to the C4 site in the chlorin structure im- and pharmacokinetics
proved the cellular internalization of C34 (Fig. 3C). Treatment of The feature of substantial enrichment into tumor sites is the premise
three-dimensional (3D) tumor spheroids with C34 and the analysis for a drug that could be applied to cancer therapy. The tumor spec-
of annexin V/propidium iodide (PI) staining after US irradiation ificity of C34 was first assessed on the xenograft H22 tumor–bearing
showed that the proportion of apoptotic and/or necrotic cells increased mouse model. C34 exhibited notable tumor-specific distribution from
from the edge to the core of tumor spheroids as time increased 1-hour intravenous post-injection assessed by in vivo real-time fluo-
(Fig. 3D), suggesting the good multilayer cellular penetrability of C34. rescence imaging system (Fig. 4A). Consistently, high-performance
In some extent, SDT drives tumor cells to go through ICD (35). As liquid chromatography (HPLC) quantitative analysis showed in-
a consequence of those mechanisms, the dying tumor cells release creased accumulation of C34 in the tumor tissues as time increased
damage-related molecular patterns and tumor antigens, thus trig- after administration (Fig. 4B), validating the excellent tumor-targeting
gering immune responses and creating an “antitumor vaccine” (35). and tumor-accumulating capacity of C34 in vivo. Similar tendency
Adenosine 5′-triphosphate (ATP) molecules released from damaged was observed in the liver but not in other organs (fig. S4 and table
cells are regarded as a “find-me” signal, causing cytokine produc- S2), suggesting that C34 might undergo the liver metabolic pathway.
tion from APCs (36, 37). The high expression of heat shock protein The prominent tumor-targeting capacity of C34 was further evaluated
70 (HSP70) under stress is referred to as a distinct biomarker of ICD on the orthotopic 4T1 breast cancer model as well. The ex vivo fluo-
as well (38). Using a luciferase-based ATP testing probe, we observed rescence imaging of the collected organs provided the direct evidence
abundant ATP secreted from the group treated with C34 + US of the localization of C34 in the tumor tissue (Fig. 4E). To investi-
(Fig. 3E). In addition, HSP70 was remarkably up-regulated in the gate the pharmacokinetics of C34, plasma samples were collected
C34-treated group at 6 hours after US irradiation (Fig. 3, F and G). and analyzed. As shown in Fig. 4C, the peaks of C34 and internal
Wang et al., Sci. Adv. 2021; 7 : eabj4796 20 October 2021 4 of 15